An increasein internal energy B. Explain why the formation of a solution is a spontaneous process.

Factors That Affect Chemical Reaction Rates Chemical Reactions Chemistry Chemistry Classroom
Melting of ice is a spontaneous process because liquid state is more random than the solid state.

. Previous question Next question. And iron will rust. The spontaneous formation of a solution is by favored by.
The formation of a solution is an example of a spontaneous process a process that occurs under specified conditions without the requirement of energy from some external source. Definition of a Spontaneous Process. See the answer See the answer See the answer done.
A spontaneous process is one that occurs naturally under certain conditions. For example a ball will roll down an incline. A homogeneous solution would form if we waited long enough.
A homogeneous solution would form if we waited long enough. Explain why the formation of a solution is a spontaneous process. For instance a sugar cube will spontaneously.
2 Dissolution of Ammonium Chloride is spontaneous because in the solid the ions are fixed but when they go into the aqueous solution they are free to move about. An increase in volume D. Water will flow downhill.
A spontaneous process is capable of proceeding in a given direction without needing to be driven by an outside source of energy. This problem has been solved. Redistribution of Matter during a Spontaneous Process.
Sometimes we stir a mixture to speed up the dissolution process but this is not necessary. A drop of food coloring added to a glass of water forms a solution with uniform color. Sometimes we stir a mixture to speed up the dissolution process but this is not necessary.
A spontaneous process at constant temperature in a closed system is accompanied by a decrease in the free energy of the system and a time. The delta H in this process is negative which means that the adsorption is a spontaneous process and it is also an exothermic process. In each case a spontaneous process took place that resulted in a more uniform distribution of matter or energy.
A decrease in volume. The Formation of Solutions. A spontaneous reaction may involve an increase or decrease in enthalpy it may involve an increase or decrease in entropy but it will always involve a decrease in free energy that is a negative ΔG.
A spontaneous process is a process that occurs without outside intervention. 1 a decrease in the internal energy of the system an exothermic change - occurs as heat is absorbed or evolvedreleased. DG 0 not spontaneous but the reverse process is spontaneous DG 0 process is in a state of equilibrium Standard Free Energy Changes Can get DGo from DGo DHo - TDSo Use DGo to predict spontaneity in the standard state Can also get values of DGo from free energies of formation.
But we are not indicating that the spontaneous reaction will definitely begin other conditions may have to be met. By opening the valve so that the gas molecules can travel to the other compartment the movement of the gas molecules. The Formation of Solutions.
Spontaneous process formation of a solution a process that occurs under specified conditions without the requirement of energy from some external source. If a process is spontaneous the reverse process is not spontaneous. Sometimes we stir a mixture to speed up the dissolution process but this is not necessary.
A spontaneous process is one that occurs on its own without any energy input from the outside. Once a spontaneous reaction begins it continues till one of the reactants is gone. Diffusion is a spontaneous process since one solute is independent of the concentration gradients of other solutes and because it involves a passive transport process which implies that energy is not expended when substances diffuse down their concentration gradient.
The formation of a solution is an example of a spontaneous process a process that occurs under specified conditions without the requirement of energy from some external source. The process is spontaneous because it is accompanied by increase of randomness. A decrease in internal enery C.
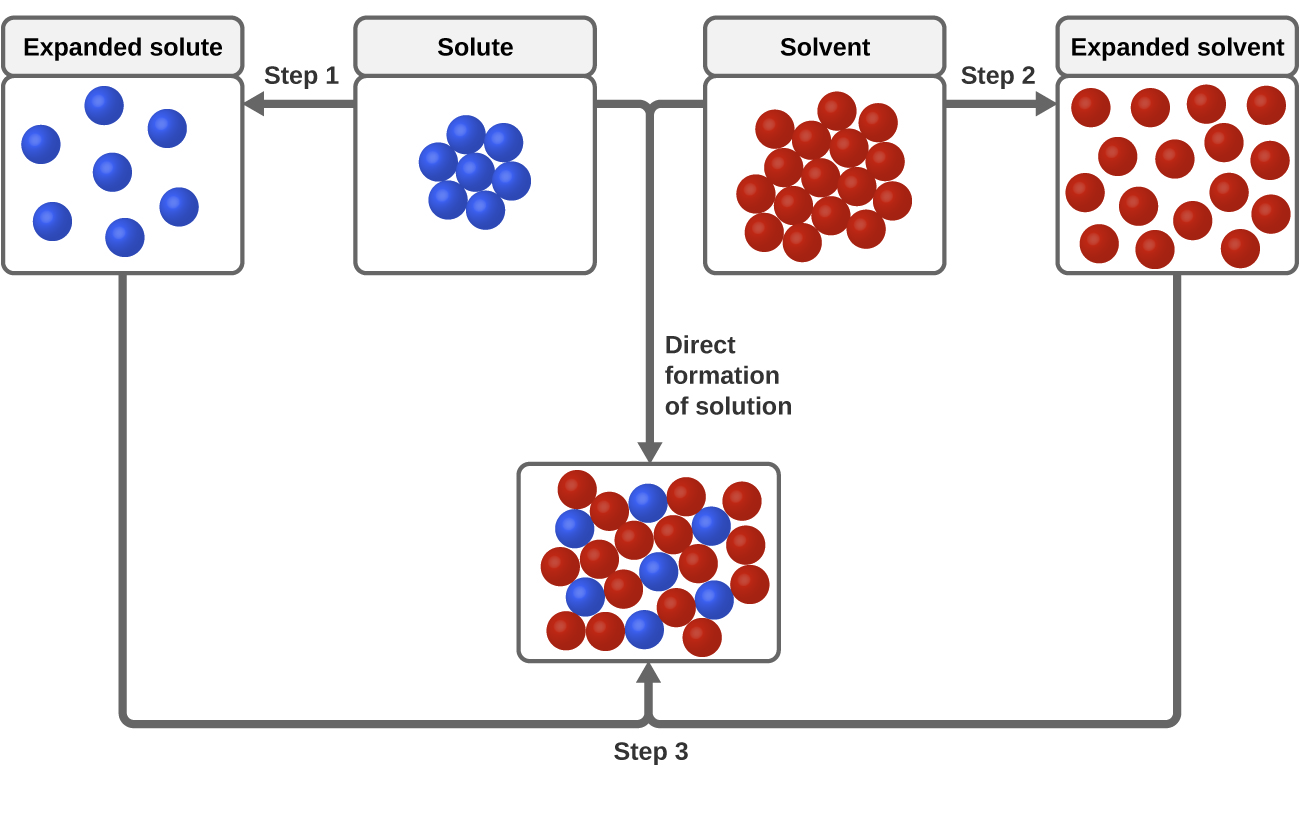
Therefore the energy of solution formation the enthalpy of solution equals the sum of the three steps. The Formation of Solutions. A process that is spontaneous in one direction under a particular set of conditions is nonspontaneous in the reverse.
Adsorption is a spontaneous process because there is a force of attraction existing between the adsorbate and adsorbent which releases heat energy. In thermodynamics a spontaneous process is a process which occurs without input of matter or electrical energy into the system. A homogeneous solution would form if we waited long enough.
Describe how matter and energy are redistributed when the following spontaneous processes take place. Show activity on this post. The expansion of gases is a spontaneous process as shown in the figure below.
What is a solute and what is a solvent. Stay tuned with BYJUS to learn more about other concepts such as the applications of. A nonspontaneous process on the other hand will not take place unless it is driven by the continual input of energy from an external source.
The formation of a solution is an example of a spontaneous process a process that occurs under specified conditions without the requirement of energy from some external source. As illustrated by the two processes described an important factor in determining the spontaneity of a process is the extent to which it changes the dispersal or distribution of matter andor energy. When we describe a reaction as spontaneous it just means that the process may occur necessary conditions are met.
Ice will melt into water. The laws of thermodynamics govern the direction of a spontaneous process ensuring that if a sufficiently large number of individual interactions are involved then the direction will always be in the direction of increased entropy. The breaking of bonds requires or absorbs energy.
Two criteria that favor the spontaneous formation of a solution. D H soln D H 1 D H 2 D H 3. DGo f formation from the elements DGo.
From Hesss law we know that we can add the energies of each step in the cycle to determine the energy of the overall process. This process is endothermic. A spontaneous process in a completely isolated system is characterized by an increase in entropy.
Enthalpy is only one of the contributing factors. Whether a given process including formation of a solution occurs spontaneously depends on whether the total energy of the system is lowered as a result.
11 1 The Dissolution Process Chemistry

Spontaneous Formation And Base Pairing Of Plausible Prebiotic Nucleotides In Water Nature Communications Molecular Biology Spontaneous Molecular

Crystals Formed In The Gelatin Matrix Sem Images Of Regular Dendrites Optical Microscope Image Of Dense Branching Morphology Credit Goes To Ryuta Ise Yuya

Determined To Succeed Chemistry Basics Chemistry Education Teaching Chemistry
0 Comments